Neuralink, a company founded by Elon Musk and engaged in the implantation of chips in the brain, said that the U.S. Food and Drug Administration (FDA) approved the first clinical trials in humans. This decision was an important milestone after a number of previous attempts to obtain permission, writes Reuters.
The FDA approval represents an important first step that will one day allow chipping to help many people, Neuralink said. The company did not specify the scope of the upcoming study and its goals, but added that the recruitment of volunteers has not yet begun. Details will follow later.
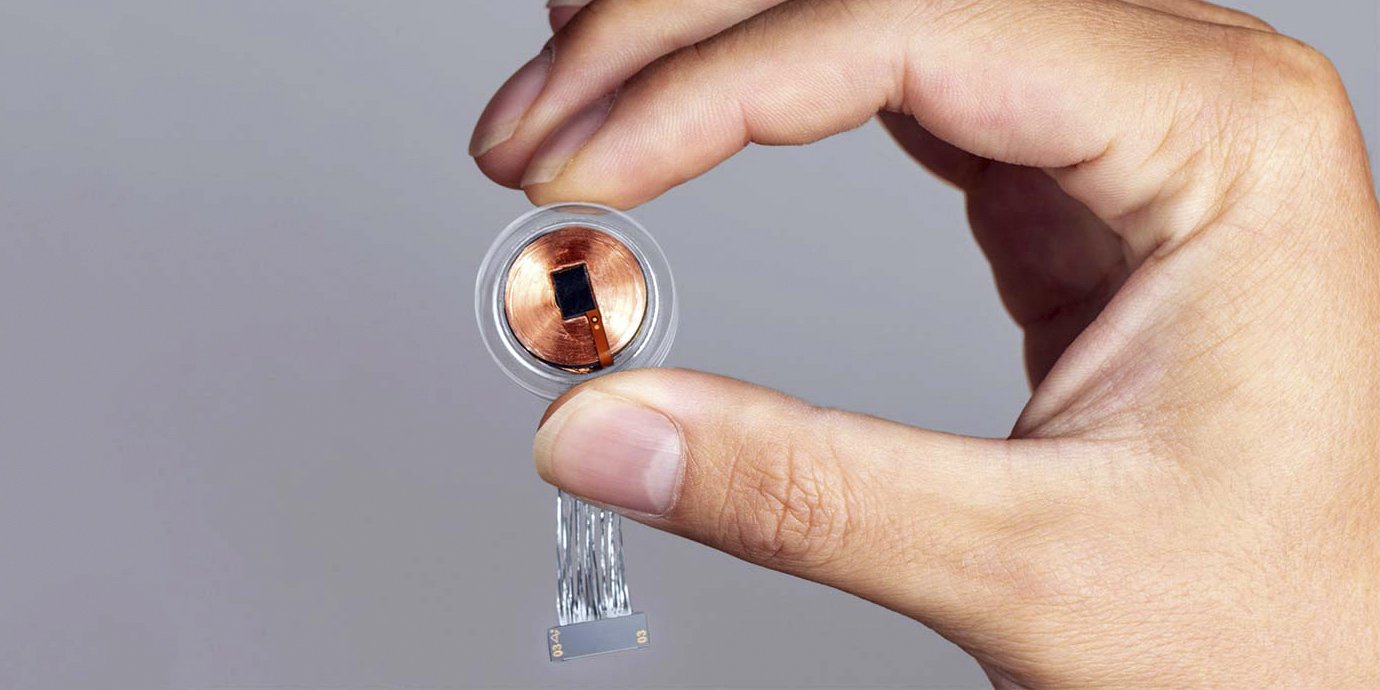
Musk is confident that brain implants will help fight a number of abnormalities in people, including obesity, autism, depression and schizophrenia, as well as treat brain and spinal cord injuries and even provide the ability to control devices with the power of thought. At the end of last year, he even stated that he was so confident in the safety of the technology that he was ready to implant chips to his children.
Neuralink has been talking about rapid human trials since 2019. But for the first time, the company requested FDA approval only in early 2022, and then the agency rejected the application.
According to employees, the FDA has pointed out several problems to Neuralink that need to be addressed before authorizing human trials. The main difficulties are related to the lithium battery of the device, the possibility of migration of the implant wires inside the brain and the problem of safely removing the chip without damaging brain tissue.
In 2019, Neuralink has already tested its chips on monkeys. In April 2021, the developers showed a video clip with a primate playing a video game with the power of thought. Animal rights activists then began to accuse Musk of cruelty to animals, because, according to them, some of the experimental monkeys died.