Hard is called Hardness in Drinking‑water: Background document for development of WHO Guidelines for Drinking‑water Quality / WHO water with a high content of mineral salts, mainly calcium and magnesium. Salts easily precipitate and create a plaque.
This plaque complicates the operation of household appliances, such as dishwashers or washing machines, and may even lead to their breakdown. And because of it, snow‑white baths and sinks quickly turn yellow, and the chrome surface of the faucets is covered with ugly stains.
The most reliable way to find out how hard water is flowing from your tap is to send a test tube with a sample of liquid for laboratory testing. You can find out the addresses of the laboratories in the help desk of your regional division of Vodokanal.
But the hardness of the water is determined independently. Here are four easy and quick ways to do it.
Pour into a glass of water, the hardness of which you want to measure. Dip a paper indicator strip with reagent squares applied to it into the liquid.

Pull out the strip and wait for about a minute (the exact duration is indicated in the instructions). Under the action of mineral salts, a chemical reaction begins, and the color on the test indicator squares will change.
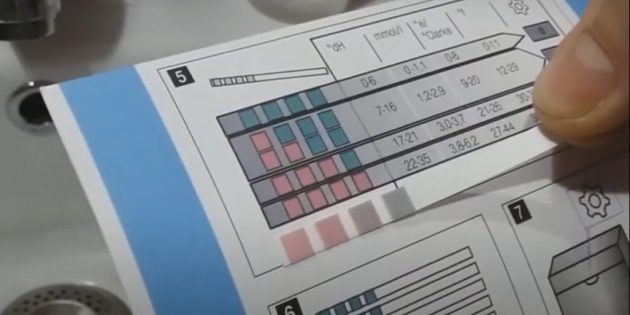
To determine the hardness of the water, compare the color in the indicator windows with the sample in the instructions.
If you use strips to determine the pH, remember the general rule: the harder the water, the more alkaline it is — that is, the higher the pH value. As a rule, soft water has a pH of less than 7, hard water is higher pH Levels in Drinking Water / ATS Environmental 8,5.
This gadget has two electrodes that need to be lowered into the water. The electrodes create a flow of electrons and record the electrical conductivity of the liquid, which is closely related to the amount of magnesium and calcium salts.
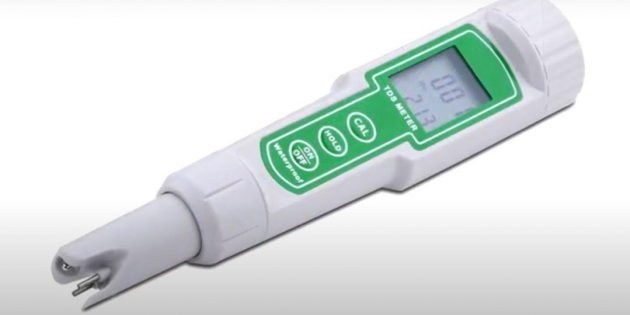
Pour the water into the glass, the hardness of which you want to measure, and lower the electrodes of the solemer into it.
The device will translate the electrical conductivity of the liquid into the units of water hardness adopted in a particular country, and show the result on the screen.
In the Russian Federation, the unit of measurement is the so—called degree of hardness (° F), it is also 1 mg‑eq. / L. The solemers certified in the Russian Federation work in this system.
Hard is considered Water hardness. Soft water. Hard water. Translation of units (degrees) water hardness. Water hardness standards. Tables of water hardness values. Water softening. How to remove water hardness / Engineering reference book. Tables DPVA.ru water with an index greater than 10 °F. A value from 2 to 10 °F corresponds to medium hardness water.
This method is based on one of the key features of hard water: it is bad Hardness in Drinking‑water: Background document for development of WHO Guidelines for Drinking‑water Quality / WHO soap foam is formed. It will require a small chemical experiment.
Rub a little household soap on a grater and measure exactly 1 g on the scales. If suddenly you do not have scales, focus on the volume: crushed soap will take about half a teaspoon without a slide.

Place the soap in a glass and add 3-4 tablespoons of warmed distilled water. Gently, so as not to form a lot of foam, stir until completely dissolved.
Then, using a ruler, add distilled water to a height (in millimeters) equal to the percentage of fatty acids in the soap. For example, if you took a standard soap with the inscription 72%, the height of the water in the glass should be 72 mm. Keep in mind that the tray of the glass also has some thickness, so measure the column of water from the bottom, and not from the surface of the table.
Gently stir the soap solution again. Carefully remove the foam if it has formed. Now there was enough soap in every centimeter of the water column to bind all the hardness salts in 1 liter of distilled water, if their concentration is 1 ° dH (German degree of hardness).
Fill 0.5 liters of cold tap water into a measuring cup or jar. Pour a thin stream of soap solution into the container and gently stir until you notice that a stable white foam has formed on the surface of the liquid. Its appearance means that the soap has completely bound the hardness salts.

Measure the height of the remaining soap solution in the glass and subtract it from the original height. So you will find out how many centimeters of the solution were poured into a measuring container.
Each centimeter of the poured solution bound in half a liter of tap water the amount of hardness salts corresponding to 2 °dH. This means that if for the formation of foam it was necessary to pour, for example, 6 cm of solution, the hardness of tap water is 12 ° dH.
To convert German degrees to those used in the Russian Federation, you can use the online calculator Water Hardness Calculator / Mosvodokanal on the website of Mosvodokanal. 12 °dH = 4.3 °W, which corresponds to medium hardness water.
This method will allow you to assume how hard the water flows from the tap. But it will not give a specific value.
Using a pipette, apply a drop of tap water and distilled water to a horizontal mirror surface. Wait until the liquid evaporates. And then analyze the remaining two spots on the glass with sediment.
The more saturated the sediment from tap water is, the more it differs from the almost imperceptible trace of distilled water, the higher the hardness.